March 2024
Special Focus: Petrochemical Technologies
A novel process for the oxidative dehydrogenation of ethane to produce ethylene and acetic acid
The authors’ company has developed a commercial chemical processa for the oxidative dehydrogenation of ethane (ODH-E). By means of a heterogeneous catalyst, ethane and oxygen are selectively converted into ethylene and acetic acid, which, after separation and purification, are provided in polymer-grade and glacial quality, respectively.
The authors’ company has developed a commercial chemical processa for the oxidative dehydrogenation of ethane (ODH-E). By means of a heterogeneous catalyst, ethane and oxygen are selectively converted into ethylene and acetic acid, which, after separation and purification, are provided in polymer-grade and glacial quality, respectively. The production of these two chemicals makes this proprietary process an excellent fit for downstream units that further process acetic acid and ethylene. This solution benefits from the authors’ company’s long-standing expertise in cryogenic and hydrocarbon gas separation. This article describes the ODH-E process and compares it with state-of-the-art ethane-steam cracking (E-SC) technology. The authors illustrate how exothermic reactions and the high selectivity to value products of this process contribute to > 60% reduction in emissions compared with the E-SC route, and how ethylene can be produced with zero Scope 1 emissions.
Background
The growing market for petrochemicals is driving demand for light olefins like ethylene, a feedstock for the manufacturing of plastics, fibers and other organic chemicals. At a compound annual growth rate (CAGR) of more than 3%, global ethylene demand is forecasted to reach approximately 230 MMt in 2028.1 Ethylene is mainly obtained by steam cracking, a well-established state-of-the-art technology with a high firing duty due to the high endothermicity of the underlying cracking reaction. This, in turn, results in high direct carbon dioxide (CO2) emissions from steam cracking in excess of 300 MMtpy,2 of which more than 75% originates from steam cracking furnaces.3 To overcome this and to reduce the carbon footprint of consumables, more sustainable ethylene production is required.
The authors’ company’s on-purpose catalytic processa is a commercial ODH-E process that converts ethane and oxygen into ethylene and acetic acid in polymer-grade and glacial quality, respectively. With less than 40% of CO2 emissions compared to the E-SC route, the process facilitates a major step toward greener ethylene and contributes to the mitigation of economic risks linked to emissions fees. The high exothermicity of the ODH-E allows the process to operate at mild temperatures (< 400°C). Furthermore, the underlaying reactions generate heat that is used by other process units, such as in the separation and purification of their value products.
C2H6 + 0.5O2 --> C2H4 + H2O ΔHR = –105 kJ/mol
C2H6 + 1.5O2 ---> C2H4O2 + H2O ΔHR = –590 kJ/mol
The on-purpose catalytic technology is an excellent fit for any ethylene-consuming processes (i.e., to produce polymers and ethylene derivatives), and also for acetic-acid-consuming downstream processes, since approximately 75% of the worldwide acetic acid demand requires additional ethylene.4 These processes include the production of vinyl acetate monomer (VAM), ethylene vinyl acetate (EVA) copolymer, polyvinyl alcohol (PVOH) products, polyethylene terephthalate (PET), ethyl acetate and similar derivatives. Compared to the commercial production of acetic acid via methanol carbonylation, the ODH-E process technology does not apply halogens—consequently, its glacial acetic acid does not inhibit the catalysts used in the downstream processes that consume acetic acid.
Catalyst and reactor features
The authors’ company has partnered with a well-known catalyst manufacturerb to develop the ODH-E catalyst. This catalyst is based on mixed-metal oxides, including molybdenum (Mo), vanadium (V), tellurium (Te) and niobium (Nb), and is produced according to patented methods.
This proprietary catalyst has been proven to have excellent stability under industrial operating conditions in the pilot plant. Based on this, the catalyst is expected to operate for more than 2 yr without a replacement. It is not regenerated in situ; instead, at the end of its lifetime, the entire volume is exchanged with a new charge. For the future, some of the metals of the replaced catalyst are set to be recycled for subsequent charges.
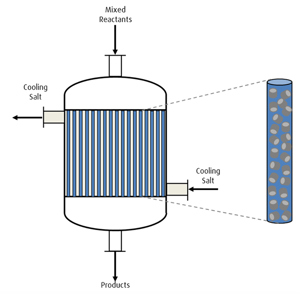
The conversion of ethane and oxygen into ethylene and acetic acid takes place in a multi-tubular reactor designed and manufactured by another partnerc of the authors’ company. This partner company has more than 60 yr of design and manufacturing experience in the field of salt-operated multi-tubular reactors, including proper and safe oxygen management and temperature control. Such reactors are applied in several industrial processes, such as the production of phthalic anhydride and maleic anhydride—i.e., in processes with similar oxidation and exothermic reactions. Uniform mixing of process steam, ethane feed and oxygen is achieved for optimum reactor performance and process safety. The design inhibits high local concentrations and flammability of the resulting ethane-oxygen-steam mixture. The mixed-reactor feed is injected into the upper section of the multi-tubular reactor (FIG. 1), where it is distributed into equal volume flows through all of the reactor tubes. The tubes are filled with catalyst pellets that facilitate the conversion of the mixed ethane and oxygen gases into the product ethylene, acetic acid, water, small amounts of carbon monoxide (CO) and CO2, and traces of acetylene.
The high exothermicity of the oxidation reactions leads to a temperature gradient along the fixed bed. Temperature control is obtained via a homogeneous cooling salt bath between the single tubes on the shell side of the reactor. The salt bath is constantly recirculated for efficient cooling and steam generation. The products leave the multi-tubular reactor at a temperature of 300°C–400°C and under moderate pressure. The maximum ethylene production per reactor is 250,000 tpy. Higher ethylene production requires the parallelization of the reactor.
The pilot plant
The ODH-E pilot plant was developed to demonstrate the process under commercial conditions. It applies a single commercial-scale reactor tube and molten-salt cooling, which is the same as that applied for the multi-tubular reactor in commercial scale. The plant consists of a reaction section and separation and purification systems. The operation of the pilot plant, which included the closed-loop recycling of purified ethane, confirmed the successful operation of the technology. In addition, the ODH-E catalyst showed very high stability through more than 6,000 hr of operation with long-term constant and varying reaction conditions. Moreover, the pilot plant confirmed that a high yield of combined ethylene and acetic acid with an overall selectivity above 93% can be achieved.
Low-emissions process plant technology
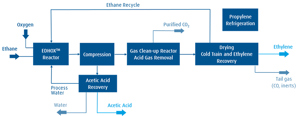
The general concept of the ODH‑E process is illustrated in FIG. 2. The plant was designed and developed to operate with ethane feedstock of pipeline specification quality that can contain small amounts of methane, residual sulfur and CO2. Fresh ethane is blended with the recycled ethane and subsequently mixed with oxygen and dilution steam before entering the reactor. The required 99.9% pure oxygen is provided by an air separation unit (outside battery limits), and the dilution steam is generated from recycled process water. The ethane is converted into ethylene and acetic acid in the multi-tubular, fixed-bed, salt bath-cooled reactor.
Downstream of the reactor, the effluent is cooled and compressed. The condensed water phase—including the formed acetic acid—is routed from the compression unit to the acetic acid recovery unit, where acetic acid of glacial purity is obtained in an extractive process. The compressed process gas undergoes the conversion of oxygen and acetylene into CO2 to ensure an oxygen-free process gas. The subsequent acid gas removal unit separates all CO2 prior to precooling and drying. In the chilling section, the process gas is cooled, separating the C1 fraction (inert methane from the feedstock, if present) and CO. The remaining C2 fraction contains ethane and ethylene and is separated into polymer-grade ethylene product and ethane, which is recycled back to the reactor.
For plant design, the ratio of the products ethylene and acetic acid can be chosen from a range of 2.5 t/t–4.5 t/t. This design point is set by the definition of reaction conditions. In this way, the design of the plant can be adjusted to best fit the requirements of the downstream processes.
Aspects of energy efficiency and CO2 emissions: ODH-E vs. E-SC
The exothermicity of the ODH-E reactions allows the operation of the chemical conversion without requiring any firing duty. Moreover, steam is generated with heat removed from the reactor and can be utilized for the separation and purification of ethylene and acetic acid. In addition, the ODH-E process is a highly selective process resulting in less byproducts compared to E-SC, and, consequently, it requires less separation efforts to gain ethylene. Through the combination of the relatively low temperature levels of the reaction, minimum heat losses and a reduced demand for heat transfer area, this process provides significant advantages with regard to investment cost and energy consumption according to typical calculation standards of energy consumption in petrochemical processes. Consequently, the specific energy consumption of the ODH-E technology is more than three times lower than that of an E-SC of the same ethylene production capacity.
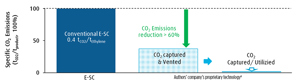
Lower energy consumption positively affects the amount of CO2 emissions—the ODH-E technology’s CO2 emissions are less than 40% vs. the E-SC route (FIG. 3). In addition, it should be noted that the CO2 byproduct generated in the proprietary process is already separated and purified (i.e., it is ready to be stored or utilized). With that, the CO2 may be used directly within battery limits for other chemical processes or conversion technologies (e.g., dry reforming or methanol synthesis). In contrast, capturing the CO2 produced from the furnace flue gas in an E-SC is not feasible within reasonable cost and effort, and, therefore, is typically not applied. Additionally, all considerations to this point do not take into account that the typical acetic acid production process based on the carbonylation of methanol also generates significant CO2 emissions.”
When the captured CO2 is further utilized or stored, the ODH-E process achieves zero Scope 1 emissions. In addition, since the process is potentially delivered as a fully electrified process, the utilization of renewable power within battery limits of the plant allows it to also achieve zero Scope 2 emissions.
Economic impact of CO2 emissions
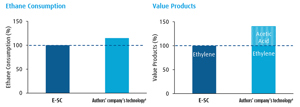
A comparison between E-SC and the ODH-E technology regarding ethane consumption and the produced value products is shown in FIG. 4. To produce the same amount of ethylene, the proprietary technology requires about 17% more ethane compared to the E-SC route. However, at the same time, the technology also provides acetic acid product, resulting in about 40% (mass basis) more valuable product and approximately 5% higher carbon efficiency.
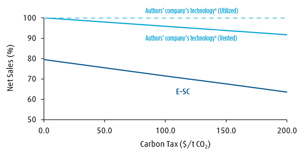
The differences concerning the carbon efficiency and CO2 emissions of E-SC and the ODH-E technology have a major impact on the net sales of both technologies. Net sales in relation to the carbon tax of both technologies are shown in FIG. 5. In the absence of a carbon tax, the net sales of E‑SC are 20% lower compared to the ODH-E technology, while a carbon tax of 200 $/t CO2 would increase the net sales difference to nearly 40%.
Takeaway
The authors’ company’s proprietary ODH-E technologya offers a sustainable catalytic solution in the conversion of ethane, generating ethylene and high-value acetic acid. The alternative oxidative route of the technology produces less byproduct than the conventional state-of-the-art E-SC technology, allowing a simplified cryogenic separation of ethylene product. In addition, it generates steam for product separation due to the exothermic reactions that take place in its multi-tubular reactor. The specific energy consumption of the ODH-E process is more than three times lower compared to E-SC of the same ethylene capacity. In turn, the proprietary technology reduces emissions by more than 60%. When the CO2 emissions directly captured from the process are utilized or stored, the proprietary process becomes a zero Scope 1 solution. Moreover, as the process is delivered fully electrified, zero Scope 2 emissions can be achieved when renewable electric power is applied. HP
NOTES
a EDHOX™ technology
b Clariant
c MAN Energy Solutions
LITERATURE CITED
1 Business Wire, “Global ethylene demand is forecasted to reach 233.9 million tons in 2028,” Research and Markets, March 28, 2022, online: https://www.businesswire.com/news/home/20220328005574/en/Global-Ethylene-Demand-is-Forecasted-to-Reach-233.9-Million-Tons-in-2028---ResearchAndMarkets.com
2 Amghizar, I., et al., “Sustainable innovations in steam cracking: CO2-neutral olefin production,” Reaction Chemistry & Engineering, 2020.
3 Vangaever, S., M. R. Djokic and K. Van Geem, “Cracking furnaces by oxy-fuel combustion, renewable energy fuels and high emissivity coatings,” AIChE Spring Meeting and Global Congress on Process Safety (virtual), 2020.
4 Sarchami, T., N. Batta and F. Berruti, “Production and separation of acetic acid from pyrolysis oil of lignocellulosic biomass: A review,” Biofuels, Bioproducts & Biorefining, August 2021.









Comments